Business Structure
Pharmaceutical companies or academic groups (genetic diseases, immune diseases, neurological diseases, etc.) who are investigating treatments for target diseases which cannot be cured by conventional modalities face the following problems when they begin to consider studying and developing oligonucleotide therapeutics.
1) They do not have any know-how to design the appropriate sequence.
2) They do not know which structures should be used.
3) Although they may try to study and develop using antisense (ASO) or siRNA therapies, they cannot delve deeply into their research due to such obstacles as ASO toxicity, siRNA biostability problems, and CMC issues.
We can solve these problems through our sequence design, by providing the most appropriate chemicals that have been selected based on their effectiveness and safety, and through selection of the most productive methods of modification and synthesis; thereby making us a key player in the development of oligonucleotide therapeutics.
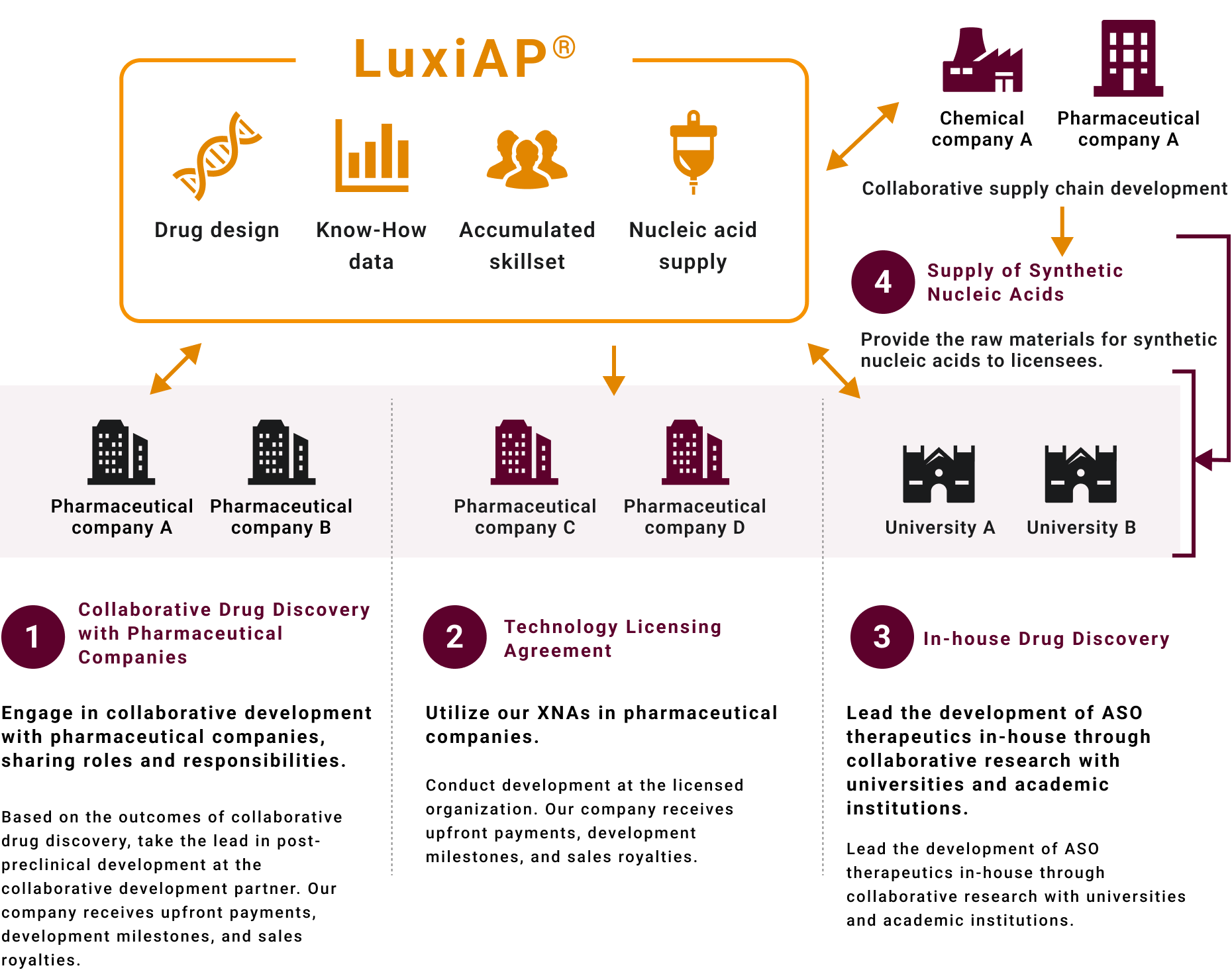

1. Collaborative Drug Discovery Program with Pharmaceutical Companies
We collaborate with pharmaceutical companies to develop ASO therapeutics for target diseases (genes).
Option 1: Starting from screening
Option 2: Starting from lead ASO optimization

2.XNAs Technology Licensing Business
For cases where there is already a resource in nucleic acid drug discovery and the use of our XNAS technology is desired, we provide gene-specific licensing.

3.In-house Drug Discovery Business
Through collaboration with academia targeting specific diseases, we aim to identify and develop lead compounds. Clinical development and beyond will be carried out by partner pharmaceutical companies.

4.XNAs Monomer Supply Business
We supply XNAs monomer units (phosphoramidites) for incorporation into ASOs through collaboration with chemical companies.

5. Others
We provide consultation services for CMC support.
